
Chemistry, 03.12.2020 20:10 harris435942
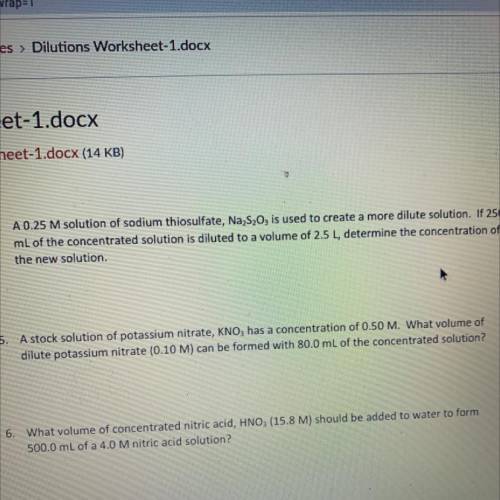
A stock solution of potassium nitrate, KNO3 has a concentration of 0.50 M. What volume of dilute potassium nitrate (0.10 M) can be formed with 80.0 mL of the concentrated solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
A stock solution of potassium nitrate, KNO3 has a concentration of 0.50 M. What volume of
dilute po...
Questions











Computers and Technology, 13.11.2019 22:31

English, 13.11.2019 22:31








English, 13.11.2019 22:31



