
Chemistry, 04.12.2020 01:00 kevonmajor
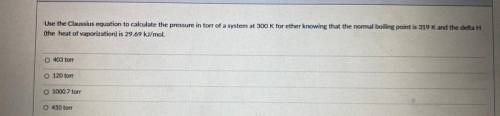
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing that the normal boiling point is 319 K and the delta H
the heat of vaporization) is 29.69 kl/mol
403 ton
120 ton
1000 7 ore
450 for

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing t...
Questions

English, 26.06.2021 22:20


Physics, 26.06.2021 22:30

English, 26.06.2021 22:30


Arts, 26.06.2021 22:30


Social Studies, 26.06.2021 22:30


Biology, 26.06.2021 22:30


Physics, 26.06.2021 22:30

Biology, 26.06.2021 22:30


Health, 26.06.2021 22:30


English, 26.06.2021 22:40


History, 26.06.2021 22:40

Health, 26.06.2021 22:40



