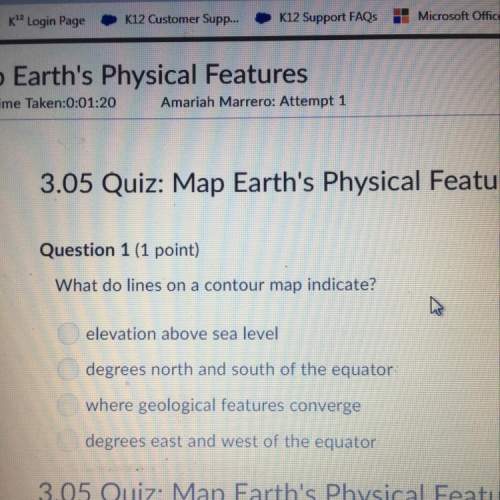
A student added 5 grams of calcium chloride, a white solid substance, to 15 grams of water at 220 C. Several observations were made and the student inferred that a chemical reaction occurred.
Which observation best supports the student's inference that a chemical reaction occurred?
A. The calcium chloride dissolved in the solution.
B. The mass of the solution totals 20 grams.
C. The temperature of the solution is 350 C.
D. The solution became lighter in color

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
A student added 5 grams of calcium chloride, a white solid substance, to 15 grams of water at 220 C....
Questions

Biology, 01.07.2019 11:00

Chemistry, 01.07.2019 11:00

History, 01.07.2019 11:00

Mathematics, 01.07.2019 11:00





Social Studies, 01.07.2019 11:00


Chemistry, 01.07.2019 11:00


Computers and Technology, 01.07.2019 11:00

Chemistry, 01.07.2019 11:00





Chemistry, 01.07.2019 11:00