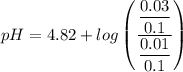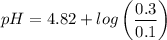Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
Calculate the pH of a buffer when 0.010 moles of NaOH is added to 100. mL solution that is 0.20 M so...
Questions




English, 09.11.2019 03:31



Mathematics, 09.11.2019 03:31

Social Studies, 09.11.2019 03:31


English, 09.11.2019 03:31

Arts, 09.11.2019 03:31


Biology, 09.11.2019 03:31



Mathematics, 09.11.2019 03:31




![pH = pKa + log \dfrac{[salt]}{[Acid]}](/tpl/images/0960/5001/27039.png)
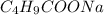 = 0.20 M
= 0.20 M 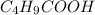 = 0.20 M
= 0.20 M