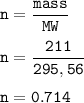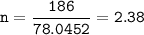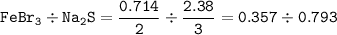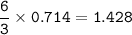A sample of 211 g of iron (III) bromide is reacted with
186 g of sodium sulfide to produce iron (III) sulfide
and sodium bromide. Using the balanced equation
below, predict which is the limiting reactant and the
maximum amount in moles of sodium bromide that
can be produced.
2FeBr3 + 3Na2S → Fe2S3 + 6NaBr

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
How many grams of naoh are needed to make 0.250 liter of a 0.500 m solution of naoh? 0.125 g 5.00 g 2.00 g
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
A sample of 211 g of iron (III) bromide is reacted with
186 g of sodium sulfide to produce iron (II...
Questions



Advanced Placement (AP), 15.12.2021 05:00



SAT, 15.12.2021 05:00

English, 15.12.2021 05:00


Engineering, 15.12.2021 05:00


Mathematics, 15.12.2021 05:00

Mathematics, 15.12.2021 05:00


Mathematics, 15.12.2021 05:00


History, 15.12.2021 05:00

English, 15.12.2021 05:00


Medicine, 15.12.2021 05:00