
Chemistry, 14.12.2020 22:50 ritasolomon85
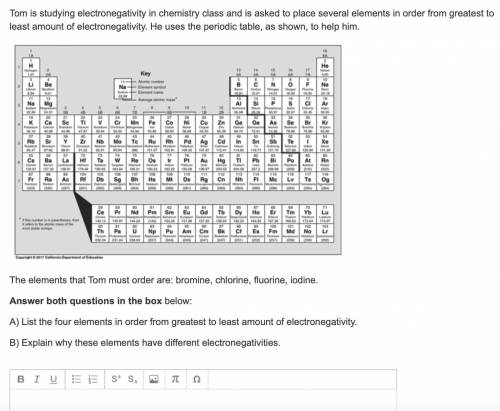
Tom is studying electronegativity in chemistry class and is asked to place several elements in order from greatest to least amount of electronegativity. He uses the periodic table, as shown, to help him.
The elements that Tom must order are: bromine, chlorine, fluorine, iodine.
Answer both questions in the box below:
A) List the four elements in order from greatest to least amount of electronegativity.
B) Explain why these elements have different electronegativities.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Tom is studying electronegativity in chemistry class and is asked to place several elements in order...
Questions

History, 29.08.2019 07:20




Chemistry, 29.08.2019 07:20

Mathematics, 29.08.2019 07:20

Mathematics, 29.08.2019 07:20




History, 29.08.2019 07:20

History, 29.08.2019 07:20

History, 29.08.2019 07:30


Mathematics, 29.08.2019 07:30

English, 29.08.2019 07:30



Mathematics, 29.08.2019 07:30



