

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
A sample of helium gas at 841 mmHg and 14.7°C is heated to 84.7°C at constant volume. Calculate its...
Questions

English, 27.11.2019 20:31

Biology, 27.11.2019 20:31


Mathematics, 27.11.2019 20:31





Mathematics, 27.11.2019 20:31

Mathematics, 27.11.2019 20:31



Mathematics, 27.11.2019 20:31



Mathematics, 27.11.2019 20:31

Mathematics, 27.11.2019 20:31

Mathematics, 27.11.2019 20:31


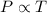 (At constant volume and number of moles)
(At constant volume and number of moles)
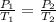
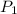 = initial pressure of gas = 841 mm Hg
= initial pressure of gas = 841 mm Hg
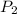 = final pressure of gas = ?
= final pressure of gas = ?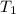 = initial temperature of gas =
= initial temperature of gas =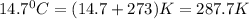
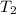 = final temperature of gas =
= final temperature of gas = 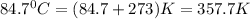
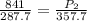
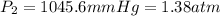 ( 760 mm Hg = 1atm )
( 760 mm Hg = 1atm )

