
Chemistry, 15.12.2020 21:50 giulianna41
Please Help!
Your car has 1.95 gallons of gasoline (octane, d = 0.6916 g/mL), which reacts with oxygen
according to the balanced reaction below. Your car uses the energy produced by this reaction
at a rate of 115 kJ per second while traveling at a speed of 65 miles per hour. Calculate the
distance (in miles) the car can travel using this amount of octane.
2 C8H18(g) + 25 O2(g) > 16 CO2(g) + 18 H2O(g) ΔHrxn = -10,900 kJ

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Please Help!
Your car has 1.95 gallons of gasoline (octane, d = 0.6916 g/mL), which reacts with oxy...
Questions

History, 13.11.2020 19:10



Mathematics, 13.11.2020 19:10

Mathematics, 13.11.2020 19:10





History, 13.11.2020 19:10



Arts, 13.11.2020 19:10



Biology, 13.11.2020 19:10


English, 13.11.2020 19:10

Geography, 13.11.2020 19:10

Biology, 13.11.2020 19:10

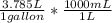 = 7380.75 mL
= 7380.75 mL

