
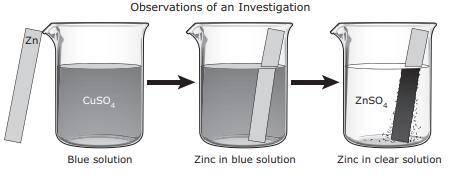
For an investigation a student poured a blue solution of CuSO4 into a beaker. The student placed a shiny, silver-colored strip of zinc metal in the solution and observed the changes. The student inferred that a chemical reaction occurred. What evidence supports this inference?
A. The CuSO4 solution turned blue when the zinc metal was added.
B. None of these
C. A dark solid formed on the zinc metal.
D. The zinc metal remained silver-colored and shiny.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
For an investigation a student poured a blue solution of CuSO4 into a beaker. The student placed a s...
Questions

Mathematics, 07.04.2020 22:16


Physics, 07.04.2020 22:16






Spanish, 07.04.2020 22:16


Mathematics, 07.04.2020 22:16

English, 07.04.2020 22:16






English, 07.04.2020 22:16


Mathematics, 07.04.2020 22:16



