
Chemistry, 18.12.2020 21:00 kingcory717
Which of the following correctly identifies the intermolecular force represented by B and compares its strength relative to the intermolecular force represented by C?
B represents ion-dipole forces, which are weaker than the force represented by C.
B represents dipole-dipole forces, which are weaker than the force represented by C.
B represents ion-dipole forces, which are stronger than the force represented by C.
B represents dipole-dipole forces, which are stronger than the force represented by C.
A concept map for four types of intermolecular forces and a certain type of bond is shown.
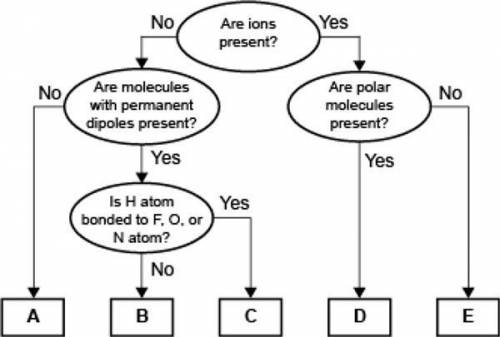

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1


Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Which of the following correctly identifies the intermolecular force represented by B and compares i...
Questions



Mathematics, 01.02.2021 16:40

English, 01.02.2021 16:40



Mathematics, 01.02.2021 16:40


Biology, 01.02.2021 16:40



Mathematics, 01.02.2021 16:40

Mathematics, 01.02.2021 16:40


Spanish, 01.02.2021 16:40

English, 01.02.2021 16:40


Mathematics, 01.02.2021 16:40





