
Chemistry, 29.12.2020 14:00 CameronVand21
What would happen to the rate of a reaction with rate law rate = k [NO]^2[H2] if
the concentration of NO were halved?
A. The rate would be four times larger.
B. The rate would also be halved.
C. The rate would be one-fourth.
D. The rate would be doubled.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
What would happen to the rate of a reaction with rate law rate = k [NO]^2[H2] if
the concentration...
Questions





Mathematics, 14.08.2019 08:30

Mathematics, 14.08.2019 08:30

Mathematics, 14.08.2019 08:30

Mathematics, 14.08.2019 08:30

Mathematics, 14.08.2019 08:30


Computers and Technology, 14.08.2019 08:30





English, 14.08.2019 08:30




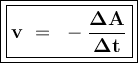
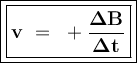
![\tt r_2=k[\dfrac{1}{2}No]^2[H_2]\\\\r_2=\dfrac{1}{4}k.[No]^2[H_2]\\\\r_2=\dfrac{1}{4}r_1](/tpl/images/1009/0129/44ffe.png)


