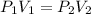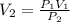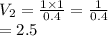Chemistry, 05.01.2021 20:20 MorallyGray
A balloon is filled with helium gas has a volume of 1.0 L at a pressure of 1.0 atm. The
balloon is released and reaches an altitude where the pressure is now 0.4 atm. What is the
new volume of the balloon at this altitude assuming the air temperature has not changed?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2
You know the right answer?
A balloon is filled with helium gas has a volume of 1.0 L at a pressure of 1.0 atm. The
balloon is...
Questions






Spanish, 02.10.2019 01:10


Mathematics, 02.10.2019 01:10


Mathematics, 02.10.2019 01:10



Mathematics, 02.10.2019 01:10


English, 02.10.2019 01:10

History, 02.10.2019 01:10

Mathematics, 02.10.2019 01:10


Mathematics, 02.10.2019 01:10