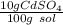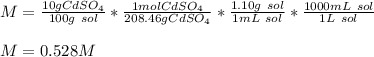Chemistry, 08.01.2021 17:10 danteyoungblood7
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol) by mass? The density of the solution is 1.10 g/mL.
a) 0.528 M
b) 0.436 M
c) 0.479 M
d) 0.048 M
e) 22.9 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)...
Questions




Physics, 25.01.2021 21:40


Social Studies, 25.01.2021 21:40




Biology, 25.01.2021 21:40





Mathematics, 25.01.2021 21:40

Business, 25.01.2021 21:40

Mathematics, 25.01.2021 21:40



History, 25.01.2021 21:40