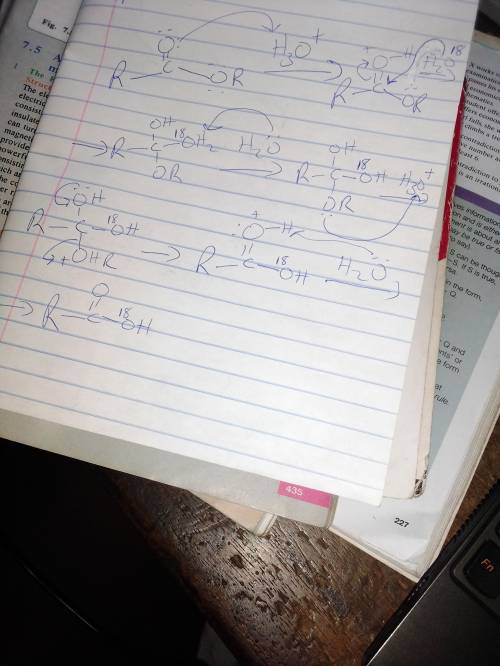Chemistry, 02.02.2021 05:20 tdahna0403
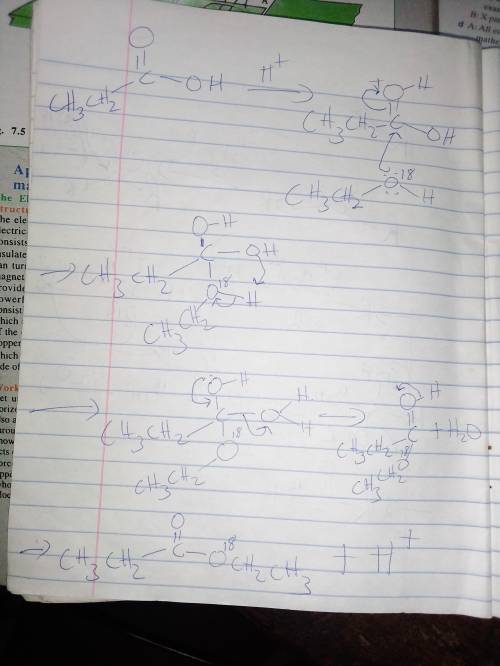
Write a mechanism for the esterification of propanoic acid with 18O-labeled ethanol. Show clearly the fate of the 18O label. (b) Acid-catalyzed hydrolysis of an unlabeled ester with 18O-labeled water (H218O) leads to incorporation of some 18O into both oxygens of the carboxylic acid product. Explain by a mechanism. (Hint: You must use the fact that all steps in the mechanism are reversible.)

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1


Chemistry, 23.06.2019 14:00
Which word refers to the smallest functional unit of living thing
Answers: 1
You know the right answer?
Write a mechanism for the esterification of propanoic acid with 18O-labeled ethanol. Show clearly th...
Questions


History, 12.02.2020 23:54




Social Studies, 12.02.2020 23:54

Mathematics, 12.02.2020 23:54

Social Studies, 12.02.2020 23:54




History, 12.02.2020 23:55





Mathematics, 12.02.2020 23:55


Mathematics, 12.02.2020 23:55