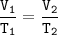Chemistry, 06.02.2021 07:50 jaimejohnston2
An arctic weather balloon is filled with 4.96 L of helium gas inside a prep shed. The temperature inside the shed is 6. °C. The balloon is then taken outside, where the temperature is 2. °C. Calculate the new volume of the balloon.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
An arctic weather balloon is filled with 4.96 L of helium gas inside a prep shed. The temperature in...
Questions



Mathematics, 02.06.2020 09:57


Mathematics, 02.06.2020 09:57

Mathematics, 02.06.2020 09:57

Physics, 02.06.2020 09:57

Mathematics, 02.06.2020 09:57

English, 02.06.2020 09:57

Mathematics, 02.06.2020 09:57

Mathematics, 02.06.2020 09:57

Mathematics, 02.06.2020 09:57

History, 02.06.2020 09:57

Biology, 02.06.2020 09:57


Chemistry, 02.06.2020 09:57

English, 02.06.2020 09:57