What is the systematic name and molecular formula of these two:
...

Chemistry, 06.02.2021 14:20 drepeter86
What is the systematic name and molecular formula of these two:
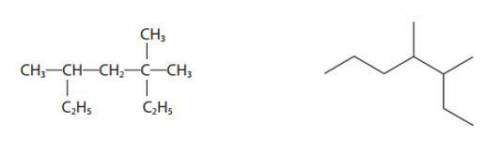

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3


Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 18:10
A1 forms when an acid is neutralized by a base. 1. salts can be neutral, or in solutions. salts of 2. strong acid–strong base reactions produce solutions with 3. water. salts formed from the neutralization of weak acids or weak 4. bases water. they produce solutions that are acidic or . basic. for example, the ph of a solution at the equivalence point is . greater than for a acid titration. solutions that resist changes in ph are called solutions. the buffer is the amount of acid or base that can be added to a buffer without changing the ph greatly.
Answers: 1
You know the right answer?
Questions




Mathematics, 01.02.2021 22:50

Physics, 01.02.2021 22:50




History, 01.02.2021 22:50


Mathematics, 01.02.2021 22:50

Mathematics, 01.02.2021 22:50


Advanced Placement (AP), 01.02.2021 22:50

Mathematics, 01.02.2021 22:50


Physics, 01.02.2021 22:50



Mathematics, 01.02.2021 22:50



