
. Carbamide (CH4N2O), can be synthesized by the reaction of ammonia (NH3) with carbon
dioxide (CO2):
2 NH3 (aq) + CO2 (aq) CH4N2O (aq) + H2O (l)
An industrial synthesis of urea obtains 87.5 kg of urea upon reaction of 68.2 kg of ammonia
with 105 kg of carbon dioxide. Determine the limiting reactant, theoretical yield of urea and percent yeild for the reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
. Carbamide (CH4N2O), can be synthesized by the reaction of ammonia (NH3) with carbon
dioxide (CO2)...
Questions

History, 08.06.2020 04:57

Mathematics, 08.06.2020 04:57

Mathematics, 08.06.2020 04:57




History, 08.06.2020 04:57











Social Studies, 08.06.2020 04:57


English, 08.06.2020 04:57

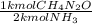 = 2.00 kmol CH₄N₂O
= 2.00 kmol CH₄N₂O

