
Chemistry, 21.02.2021 23:30 ExclusiveNay
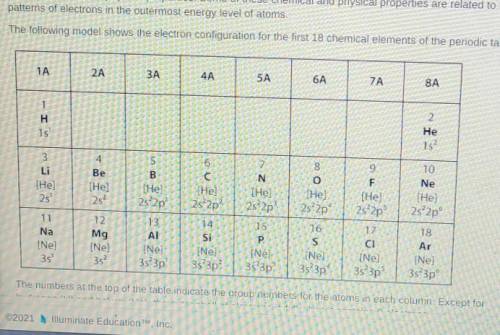
Which statement best explains the type of bond that will form between two elements from group 6A in the model?
A. The two elements will form a covalent bond because both elements will share a single electron in order to have full outer shells
B. The two elements will form a covalent bond because both elements will share a pair of electrons in order to have full outer shells.
C. The two elements will form an ionic bond because one of the elements will donate one electron to the other element in order to have full outer shells.
D. The two elements will form an ionic bind because one of the elements will donate two electrons to the other elements in order to have full outer shells.
*Will give brainliest*

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1
You know the right answer?
Which statement best explains the type of bond that will form between two elements from group 6A in...
Questions


Spanish, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00


Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00



Social Studies, 05.05.2021 01:00



Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Geography, 05.05.2021 01:00



Mathematics, 05.05.2021 01:00





