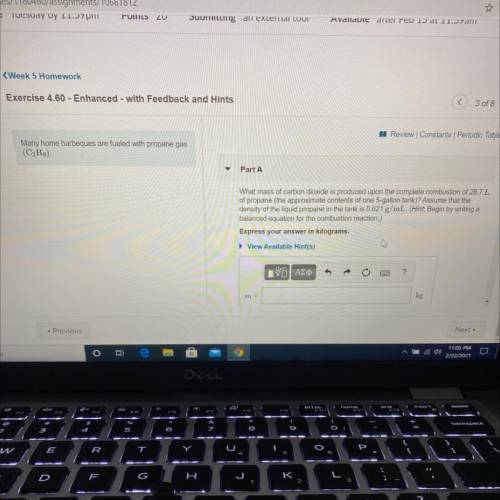
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is...

Chemistry, 23.02.2021 09:20 keilajohnson810
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is produced upon the complete combustion of 28.7L
of propane (the approximate contents of one 5-gallon tank)? Assume that the
density of the liquid propane in the tank is 0.621 g/mL.
Express your answer in kilograms.
Will give brainliest!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Questions





English, 09.12.2020 14:00

Advanced Placement (AP), 09.12.2020 14:00



History, 09.12.2020 14:00


Arts, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00


Computers and Technology, 09.12.2020 14:00



History, 09.12.2020 14:00



