Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3
You know the right answer?
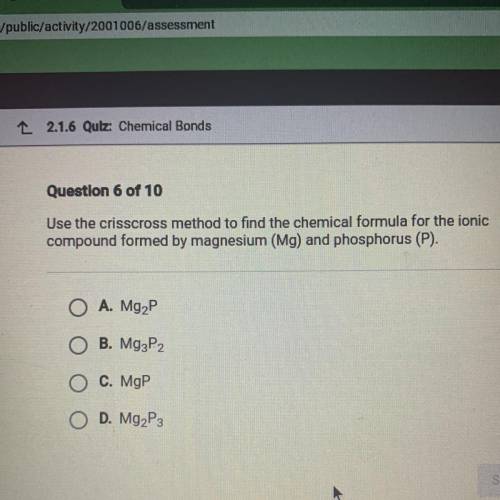
(Use the crisscross method to find the chemical formula for the ionic compound formed by magnesium “...
Questions



Computers and Technology, 11.03.2020 05:37


Biology, 11.03.2020 05:37


Business, 11.03.2020 05:37



Mathematics, 11.03.2020 05:37


History, 11.03.2020 05:37

Mathematics, 11.03.2020 05:37



Computers and Technology, 11.03.2020 05:37


English, 11.03.2020 05:37