
Chemistry, 26.02.2021 23:00 mariamalakozay603
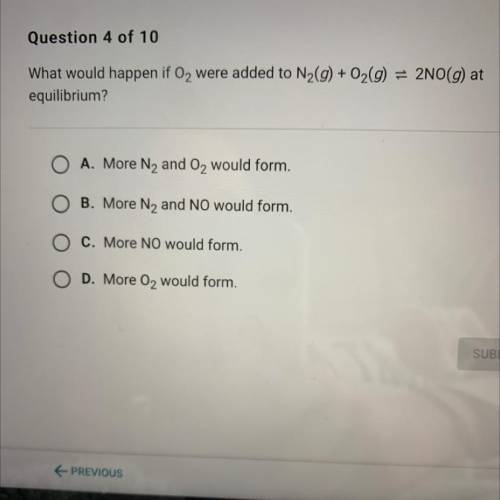
What would happen if O2 were added to N2(g) + O2(g) = 2NO(g) at equilibrium
(Answers are on the picture)
Thank you

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
What would happen if O2 were added to N2(g) + O2(g) = 2NO(g) at equilibrium
(Answers are on the pic...
Questions



Chemistry, 31.03.2021 01:00

Spanish, 31.03.2021 01:00

English, 31.03.2021 01:00


Social Studies, 31.03.2021 01:00






Mathematics, 31.03.2021 01:00


Mathematics, 31.03.2021 01:00




Mathematics, 31.03.2021 01:00



