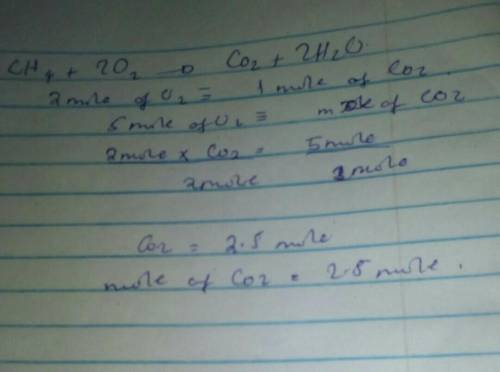The equation for the combustion of methane is
CH4+ 2O2——->CO2+2H2O
if you have 5 mol...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Questions




Computers and Technology, 21.12.2019 04:31







Computers and Technology, 21.12.2019 04:31








Mathematics, 21.12.2019 04:31

English, 21.12.2019 04:31