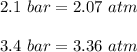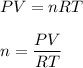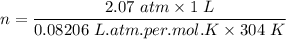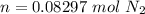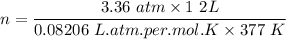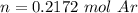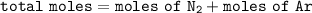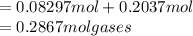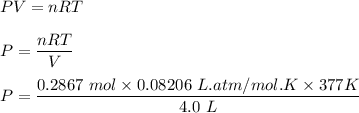Chemistry, 12.03.2021 15:30 Yasminl52899
One liter of N (g) at 2.1 bar and two liters of Ar(g) at 3.4 bar are mixed in a 4.0-L 2 flask to form an ideal-gas mixture. Calculate the value of the final pressure of the mixture if the initial and final temperature of the gases are the same. Repeat this calculation if the initial temperatures of the N (g) and Ar(g) are 304 K and 402 K, respectively, and the final 2 temperature of the mixture is 377 K. (Assume ideal-gas behavior.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1


Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
One liter of N (g) at 2.1 bar and two liters of Ar(g) at 3.4 bar are mixed in a 4.0-L 2 flask to for...
Questions



Mathematics, 02.12.2020 01:00

English, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00




Mathematics, 02.12.2020 01:00

English, 02.12.2020 01:00



Chemistry, 02.12.2020 01:00

English, 02.12.2020 01:00

English, 02.12.2020 01:00




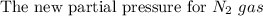
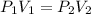
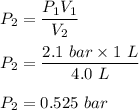
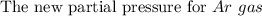
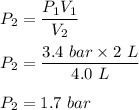
![Total pressure= P [N_2] + P[Ar] \ \\ \\ . \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ = (0.525 + 1.7)Bar \\ \\ . \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ = 2.225 \ Bar](/tpl/images/1191/0157/39a5e.png)