Chemistry, 12.03.2021 15:30 alisonlebron15
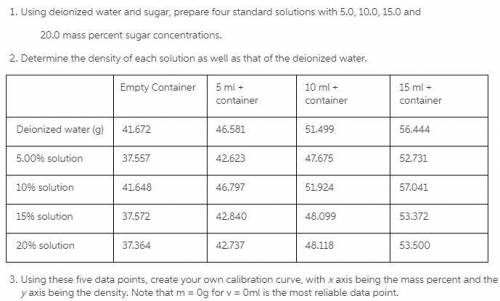
Using deionized water and sugar, prepare four standard solutions with 5.0, 10.0, 15.0 and 20.0 mass percent sugar concentrations. Determine the density of each solution as well as that of the deionized water.
Empty Container 5 ml + container 10 ml + container 15 ml + container
Deionized water (g) 41.672 46.581 51.499 56.444
5.00% solution 37.557 42.623 47.675 52.731
10% solution 41.648 46.797 51.924 57.041
15% solution 37.572 42.840 48.099 53.372
20% solution 37.364 42.737 48.118 53.500
Using these five data points, create your own calibration curve, with x axis being the mass percent and the y axis being the density.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

You know the right answer?
Using deionized water and sugar, prepare four standard solutions with 5.0, 10.0, 15.0 and 20.0 mass...
Questions


Mathematics, 13.10.2020 06:01

Biology, 13.10.2020 06:01


Biology, 13.10.2020 06:01


Social Studies, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01

English, 13.10.2020 06:01

Physics, 13.10.2020 06:01



Mathematics, 13.10.2020 06:01


History, 13.10.2020 06:01




History, 13.10.2020 06:01