

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
The concentration of Mg2+ in seawater is 0.052 M. At what pH will 76% of the Mg2+ be precipitated as...
Questions



Mathematics, 13.10.2020 20:01

Mathematics, 13.10.2020 20:01


Arts, 13.10.2020 20:01



History, 13.10.2020 20:01


Mathematics, 13.10.2020 20:01


Social Studies, 13.10.2020 20:01

English, 13.10.2020 20:01


English, 13.10.2020 20:01

Mathematics, 13.10.2020 20:01



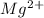 will be precipitated as the hydroxide salt.
will be precipitated as the hydroxide salt.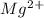 has precipitated out , 24% remains out.
has precipitated out , 24% remains out.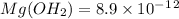
![[Mg^{2}^{+}] =\frac{24}{100}\times 0.052](/tpl/images/1200/9004/3cfbf.png)

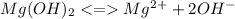
![Ksp = [Mg^2^+][OH^-]](/tpl/images/1200/9004/4a0be.png)
![8.9\times 10^-^1^2= 1.248\times10^-^2\times[OH]^-](/tpl/images/1200/9004/8a6a7.png)
![[OH]^- = 7.131\times 10^-^1^0](/tpl/images/1200/9004/e943a.png)
![[H^+]=\frac{Kw}{[OH]^-}](/tpl/images/1200/9004/234a2.png) ( where Kw is the ionic product of the water)
( where Kw is the ionic product of the water)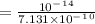

![pH=-Log[H^+]](/tpl/images/1200/9004/3ca39.png)
![-Log[1.402\times10^-^5]](/tpl/images/1200/9004/c76b6.png)


