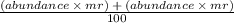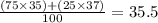Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1


Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3
You know the right answer?
Chlorine has two isotopes, Cl-35 and Cl-37 with the average atomic mass 35.5. what is the percentage...
Questions



Spanish, 01.02.2020 00:55



Mathematics, 01.02.2020 00:55

Mathematics, 01.02.2020 00:55

Geography, 01.02.2020 00:55



Mathematics, 01.02.2020 00:55






Mathematics, 01.02.2020 00:56

Health, 01.02.2020 00:56