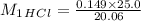Chemistry, 28.03.2021 01:00 hollycoleman13
A 20.06-mL sample of hydrochloric acid solution requires 25.00 mL of 0.149 M sodium hydroxide for complete neutralization. What is the concentration of the original hydrochloric acid solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

You know the right answer?
A 20.06-mL sample of hydrochloric acid solution requires 25.00 mL of 0.149 M sodium hydroxide for co...
Questions

Computers and Technology, 07.04.2020 21:48





Mathematics, 07.04.2020 21:48

Mathematics, 07.04.2020 21:48

Mathematics, 07.04.2020 21:48


History, 07.04.2020 21:48







Mathematics, 07.04.2020 21:48

Chemistry, 07.04.2020 21:48


 For titration between NaOH and HCl
For titration between NaOH and HCl