
Chemistry, 29.03.2021 02:20 klivingston1012
PLEASE HELP ME YALL I NEED TO GET THIS RIGHT
A chemist wants to extract copper metal from copper chloride solution. The chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (II) chloride. A single replacement reaction takes place. Which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Unbalanced equation: CuCl2 + Al → AlCl3 + Cu (4 points)
a. Approximately 0.36 grams, because copper (II) chloride acts as a limiting reactant
b. Approximately 1.8 grams, because copper (II) chloride acts as a limiting reactant
c. Approximately 0.36 grams, because aluminum acts as a limiting reactant
d. Approximately 1.8 grams, because aluminum acts as a limiting reactant

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
PLEASE HELP ME YALL I NEED TO GET THIS RIGHT
A chemist wants to extract copper metal from copper ch...
Questions










Mathematics, 27.02.2020 23:53

Mathematics, 27.02.2020 23:53




Computers and Technology, 27.02.2020 23:53



Computers and Technology, 27.02.2020 23:54


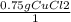 ×
×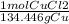 ×
×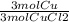 ×
×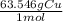 ≈0.35
≈0.35


