
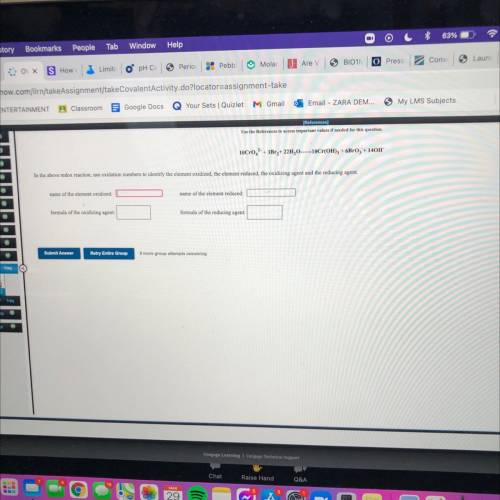
10CrO2 + 3Br + 22H010Cr(OH)3 + 6Broz + 140H
In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent.
name of the element oxidized:
name of the element reduced:
formula of the oxidizing agent:
formula of the reducing agent:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
10CrO2 + 3Br + 22H010Cr(OH)3 + 6Broz + 140H
In the above redox reaction, use oxidation numbers to i...
Questions

Computers and Technology, 25.03.2020 00:01

History, 25.03.2020 00:01

Mathematics, 25.03.2020 00:01

Mathematics, 25.03.2020 00:01

Mathematics, 25.03.2020 00:01


Computers and Technology, 25.03.2020 00:01





English, 25.03.2020 00:02

History, 25.03.2020 00:02


Mathematics, 25.03.2020 00:02

Mathematics, 25.03.2020 00:02



Social Studies, 25.03.2020 00:02




