
Chemistry, 11.04.2021 23:30 sarahelisabeth444
Help please
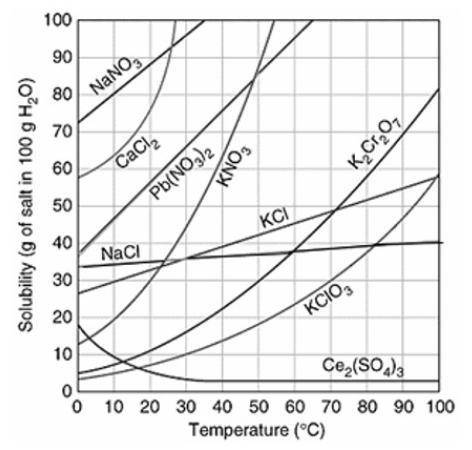
Assuming that the trends continue, which of the following compounds do you predict will have the GREATEST solubility at 120°C?
A.
Ce2(SO4)3
B.
K2Cr2O7
C.
Pb(NO3)2
D.
NaCl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
Help please
Assuming that the trends continue, which of the following compounds do you predict will...
Questions

Chemistry, 18.11.2020 15:30


Chemistry, 18.11.2020 15:30


Chemistry, 18.11.2020 15:30

Mathematics, 18.11.2020 15:40

English, 18.11.2020 15:40

Social Studies, 18.11.2020 15:40

Chemistry, 18.11.2020 15:40

Social Studies, 18.11.2020 15:40

History, 18.11.2020 15:40

Mathematics, 18.11.2020 15:40




English, 18.11.2020 15:40






