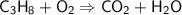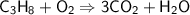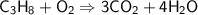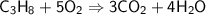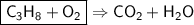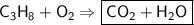This is the equation for the combustion of propane.
c3h8 + 5o2 → 3co2 + 4h2o + heat
which are the reactants and the products in this reaction?
a.
the reactants are c3h8 (propane) and h2o (water). the products are o2 (oxygen) and co2 (carbon dioxide).
b.
the reactants are c3h8 (propane) and o2 ( oxygen). the products are co2 (carbon dioxide) and h2o (water).
c.
the reactants are co2 (carbon dioxide) and h2o (water). the products are c3h8 (propane) and o2 ( oxygen).
d.
the reactants are o2 (oxygen) and co2 (carbon dioxide). the products are c3h8 (propane) and h2o (water).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
This is the equation for the combustion of propane.
c3h8 + 5o2 → 3co2 + 4h2o + heat
whic...
c3h8 + 5o2 → 3co2 + 4h2o + heat
whic...
Questions




Mathematics, 31.08.2019 05:30

Health, 31.08.2019 05:30


Mathematics, 31.08.2019 05:30

Mathematics, 31.08.2019 05:30



Mathematics, 31.08.2019 05:30


Mathematics, 31.08.2019 05:30

Computers and Technology, 31.08.2019 05:30

Mathematics, 31.08.2019 05:30

Medicine, 31.08.2019 05:30

English, 31.08.2019 05:30




![\rule[225]{225}{2}](/tpl/images/0178/4900/85973.png)