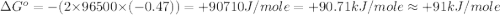Chemistry, 31.08.2019 05:30 elizabethcswind5062
Use the provided reduction potentials to calculate argº for the following balanced redox reaction: pb2+(aq) + cu(s) → pb(s) + cu2+(aq) e°(pb2+/pb) = -0.13 v and e°(cu2+/cu) = +0.34 v -41 kj mol-1 +91 kj mol-1 -21 kj mol-1 -0.47 kj mol-1 +46 kj mol-1

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Use the provided reduction potentials to calculate argº for the following balanced redox reaction:...
Questions



Mathematics, 26.02.2020 19:56

Mathematics, 26.02.2020 19:56

History, 26.02.2020 19:56




Mathematics, 26.02.2020 19:56








Mathematics, 26.02.2020 19:56



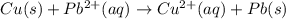
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0214/1462/82211.png)
![E^0_{[Cu^{2+}/Cu]}=+0.34V](/tpl/images/0214/1462/ecde5.png)
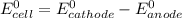
![E^0_{cell}=E^0_{[Pb^{2+}/Pb]}-E^0_{[Cu^{2+}/Cu]}](/tpl/images/0214/1462/6ec7d.png)
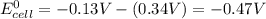
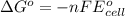
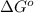 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?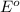 = standard e.m.f of cell = -0.47 V
= standard e.m.f of cell = -0.47 V