
Chemistry, 18.04.2021 01:00 raquelle66
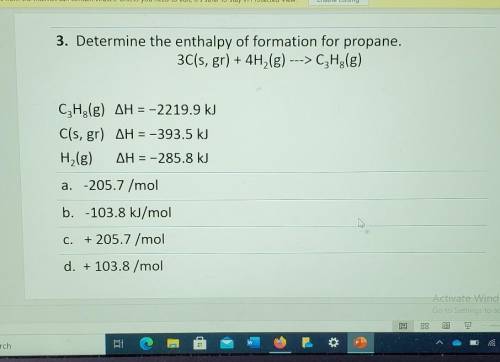
3. Determine the enthalpy of formation for propane. 3C(s, gr) + 4H2(g) ---> C3H2(g) CzH3(g) AH = -2219.9 kJ C(s, gr) AH = -393.5 kJ H,(g) AH = -285.8 kJ a. -205.7 /mol b. -103.8 kJ/mol Å C. + 205.7 /mol d. + 103.8 /mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

You know the right answer?
3. Determine the enthalpy of formation for propane. 3C(s, gr) + 4H2(g) ---> C3H2(g) CzH3(g) AH =...
Questions



Chemistry, 26.12.2019 22:31




Social Studies, 26.12.2019 22:31















