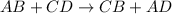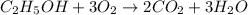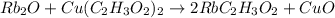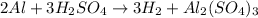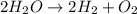Chemistry, 19.09.2019 01:00 seider7997
Which reaction below represents a balanced, double replacement chemical reaction? a) c2h5oh + 3o2 → 2co2 + 3h2o b) rb2o + cu(c2h3o2)2 → 2rbc2h3o2 + cuo c) 2al + 3h2so4 → 3h2 + al2(so4)3 eliminate d) 2h2o → 2h2 + o2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1


Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
You know the right answer?
Which reaction below represents a balanced, double replacement chemical reaction? a) c2h5oh + 3o2 →...
Questions











Mathematics, 25.11.2019 21:31