
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2o → oh- + sh- which of the following statements is true? na2s is a base because it ionizes to release oh-. na2s is an acid because it is a proton donor. na2s is a base because it increases the hydroxide concentration. none of these

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1


Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2...
Questions

Mathematics, 22.11.2020 23:10


Mathematics, 22.11.2020 23:10




Mathematics, 22.11.2020 23:20




English, 22.11.2020 23:20

Biology, 22.11.2020 23:20


Biology, 22.11.2020 23:20


Physics, 22.11.2020 23:20

Biology, 22.11.2020 23:20

Mathematics, 22.11.2020 23:20

Engineering, 22.11.2020 23:20

English, 22.11.2020 23:20

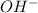 ) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution. 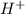 ) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.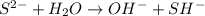
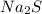 ) dissociates to give hydroxide ions.
) dissociates to give hydroxide ions. 

