
Chemistry, 05.02.2020 11:46 jonathanjenkins701
How many liters of a 2.25 molar hydrobromic acid (hbr) solution would be needed to react completely with 100.0 grams of calcium metal?
ca (s) + 2hbr (aq) cabr2 (aq) + h2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
How many liters of a 2.25 molar hydrobromic acid (hbr) solution would be needed to react completely...
Questions

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00



Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00




Mathematics, 15.12.2020 01:00


English, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00

Spanish, 15.12.2020 01:00


 ....(1)
....(1)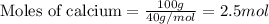
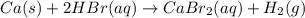
 of hydrogen bromide.
of hydrogen bromide.
 = 5 moles
= 5 moles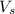 = volume of solution in liter = ?
= volume of solution in liter = ?



