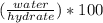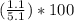Chemistry, 05.02.2020 13:01 andregijoe41
Asample of a hydrated compound has a mass of 5.1 g. during heating, it loses 1.1 g, leaving 4.0 g. what is the percentage by mass of water in the original hydrate?
6.11%
21.6%
27.5%
78.4%

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 23.06.2019 08:00
Why is it important for scientists to review and repeat the work of other scientists? 1.a scientific theory must be tested three times before it is proven. 2.the scientific method only applies to repeated experiments. 3.an experiment may have had errors that the scientists didn't recognize. 4.the results of individual scientists may be influenced by bias. 5.an experiment must be performed twice before the data can be analyzed.
Answers: 3
You know the right answer?
Asample of a hydrated compound has a mass of 5.1 g. during heating, it loses 1.1 g, leaving 4.0 g. w...
Questions

Geography, 30.11.2021 19:10



Law, 30.11.2021 19:10

Mathematics, 30.11.2021 19:10



Social Studies, 30.11.2021 19:10

Mathematics, 30.11.2021 19:10


Arts, 30.11.2021 19:10



Mathematics, 30.11.2021 19:10






Mathematics, 30.11.2021 19:20