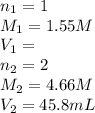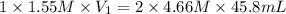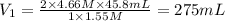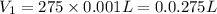Chemistry, 28.08.2019 04:30 diegobebe503
For the neutralization reaction involving hno3 and ca(oh)2, how many liters of 1.55 m hno3 are needed to react with 45.8 ml of a 4.66 m ca(oh)2 solution?
1. 0.137 l 2. 0.0343 l 3. 0.275 l 4. 1.32 l 5. 0.662 l 6. 0.330 l

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
For the neutralization reaction involving hno3 and ca(oh)2, how many liters of 1.55 m hno3 are neede...
Questions





English, 14.06.2020 03:57





Mathematics, 14.06.2020 03:57

Mathematics, 14.06.2020 03:57

Mathematics, 14.06.2020 03:57


Biology, 14.06.2020 03:57

Biology, 14.06.2020 03:57


History, 14.06.2020 03:57


Mathematics, 14.06.2020 03:57

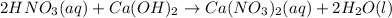
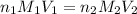
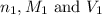 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 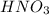
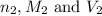 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 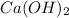 .
.