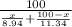Chemistry, 03.02.2020 07:56 stinematesa
Abag contains a mixture of copper and lead bbs. the average density of the bbs is 9.60g/cm3.assuming that the copper and lead are pure, determine the relative amounts of each kind of bb.
express your answers numerically separated by a comma.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1


Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

You know the right answer?
Abag contains a mixture of copper and lead bbs. the average density of the bbs is 9.60g/cm3.assuming...
Questions

Chemistry, 02.09.2021 19:20


Physics, 02.09.2021 19:20


History, 02.09.2021 19:20


English, 02.09.2021 19:20

Mathematics, 02.09.2021 19:20




Mathematics, 02.09.2021 19:20







Mathematics, 02.09.2021 19:20

Mathematics, 02.09.2021 19:20

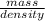
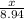
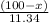
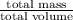
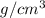 =
=