
Chemistry, 02.10.2019 21:00 claudia122752
Calculate the number of moles of excess reactant that will be left-over when 50.0 g of ki react with 50.0 g of br2: 2ki + br2 2kbr + i2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Calculate the number of moles of excess reactant that will be left-over when 50.0 g of ki react with...
Questions


Social Studies, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30




Mathematics, 12.12.2020 16:30

Business, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Spanish, 12.12.2020 16:30



Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Chemistry, 12.12.2020 16:30

Geography, 12.12.2020 16:30

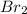 is, 0.1625 mole.
is, 0.1625 mole.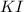 = 50 g
= 50 g
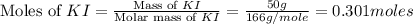
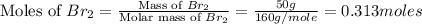
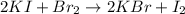
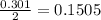 moles of
moles of 

