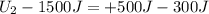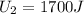Chemistry, 31.01.2020 22:43 jackie6852
In a heat engine, if 500 j of heat enters the system, and the piston does 300 j of work, what is the final internal (thermal) energy of the system if the initial energy is 1,500 j?
800 j
1,300 j
200 j
1,700 j

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
In a heat engine, if 500 j of heat enters the system, and the piston does 300 j of work, what is the...
Questions



Mathematics, 06.02.2021 07:40



Mathematics, 06.02.2021 07:40

Arts, 06.02.2021 07:40


Business, 06.02.2021 07:40

Mathematics, 06.02.2021 07:40


Health, 06.02.2021 07:40

Social Studies, 06.02.2021 07:40

Mathematics, 06.02.2021 07:50

Chemistry, 06.02.2021 07:50


Mathematics, 06.02.2021 07:50



Mathematics, 06.02.2021 07:50

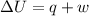
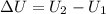 =Final energy-initial energy=Change in internal energy
=Final energy-initial energy=Change in internal energy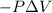 {Work done by the system is negative as the final volume is greater than initial volume}
{Work done by the system is negative as the final volume is greater than initial volume}