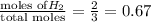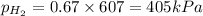Abox with a volume of 11.2 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0°c which of the following statements is true?
r = 8.31 kpa x l/mol x k, pv = nrt
a. the partial pressures of n₂ and h₂ are equal.
b. the partial pressure of n₂ is 202 kpa.
c. the total pressure is 303 kpa.
d. the total pressure in the box is 202 kpa.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

You know the right answer?
Abox with a volume of 11.2 l contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0°c which of th...
Questions

English, 07.10.2020 21:01

Geography, 07.10.2020 21:01

Chemistry, 07.10.2020 21:01

English, 07.10.2020 21:01

Mathematics, 07.10.2020 21:01


English, 07.10.2020 21:01



Mathematics, 07.10.2020 21:01

Social Studies, 07.10.2020 21:01



Computers and Technology, 07.10.2020 21:01

Biology, 07.10.2020 21:01


Social Studies, 07.10.2020 21:01

Chemistry, 07.10.2020 21:01


Mathematics, 07.10.2020 21:01

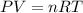
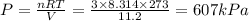
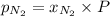
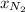 = mole fraction=
= mole fraction= 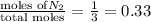
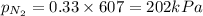
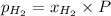
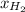 = mole fraction=
= mole fraction=