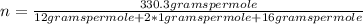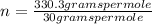Chemistry, 08.01.2020 22:31 jet0120996
The empirical formula for sucrose is ch2o. the molar mass of sucrose is 330.3 grams per mole. determine the molecular formula of sucrose.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
The empirical formula for sucrose is ch2o. the molar mass of sucrose is 330.3 grams per mole. determ...
Questions



Computers and Technology, 03.02.2021 07:00

Social Studies, 03.02.2021 07:00

Computers and Technology, 03.02.2021 07:00




Geography, 03.02.2021 07:00


Mathematics, 03.02.2021 07:00



Mathematics, 03.02.2021 07:00


English, 03.02.2021 07:00



Health, 03.02.2021 07:00