
Chemistry, 28.01.2020 21:58 alaina3792
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale for doing this is that the sodium bicarbonate is:
a base and will neutralize the excess acid
an acid and will weaken the excess acid
neutral and will dilute the excess acid
it will make the person thirsty; they will drink water and dilute the excess acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale f...
Questions

History, 27.07.2019 17:00


Health, 27.07.2019 17:00



Physics, 27.07.2019 17:00



Mathematics, 27.07.2019 17:00






Social Studies, 27.07.2019 17:00


Physics, 27.07.2019 17:00



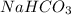 . It is basic in nature as it dissociates to give sodium and bicarbonate ions.
. It is basic in nature as it dissociates to give sodium and bicarbonate ions. 

