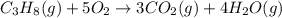Chemistry, 16.09.2019 15:50 conyabrew82
Propane camping stoves produce heat by the combustion of gaseous propane (c3h8). balance the skeletal equation for the combustion of propane.
c3h8(g)+o2(g)→co2(g)+h2o(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1


Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Propane camping stoves produce heat by the combustion of gaseous propane (c3h8). balance the skeleta...
Questions


Mathematics, 28.06.2019 20:00




Social Studies, 28.06.2019 20:00

Mathematics, 28.06.2019 20:00

English, 28.06.2019 20:00

Physics, 28.06.2019 20:00


Mathematics, 28.06.2019 20:00




World Languages, 28.06.2019 20:00

Mathematics, 28.06.2019 20:00





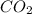 by 3 and
by 3 and  by 4 on product side by 2. Hence, the complete balanced chemical equation will be as follows.
by 4 on product side by 2. Hence, the complete balanced chemical equation will be as follows.