
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values for free energy and enthalpy at 25.0°c.
g = 130.5 kj/mol
h = 178.3 kj/mol
what is the entropy of the reaction? use g = h – ts.
a. -160.3 j/(mol. k)
b. -47.8 j/(mol. k)
c. 160.3 j/(mol. k)
d. 1,912 j/(mol. k)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Why are gamma rays not affected by a magnet as they pass over it? gamma rays are composed of only energy. gamma rays do not have enough mass to be affected. gamma rays do not have the right electrical charge to be affected. gamma rays move too fast for anything to affect their pathway.
Answers: 1


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values f...
Questions


Mathematics, 28.04.2021 17:40

English, 28.04.2021 17:40



Mathematics, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40





Mathematics, 28.04.2021 17:40

Chemistry, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40

English, 28.04.2021 17:40

Advanced Placement (AP), 28.04.2021 17:40




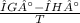
 = 0.1603kJ/(mol.K) = 160.3kJ/(mol.K)
= 0.1603kJ/(mol.K) = 160.3kJ/(mol.K) 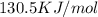

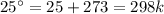
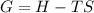
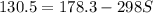

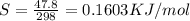
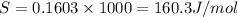 (1KJ=1000J)
(1KJ=1000J)

