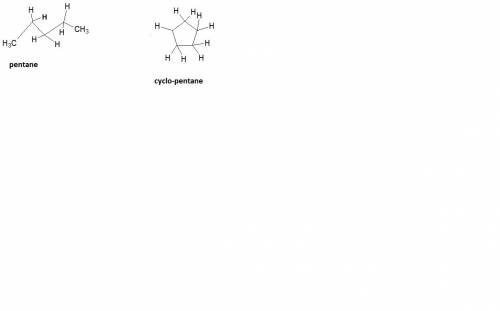
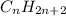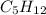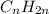How does a straight-chain alkane that has five carbon atoms differ from a cycloalkane that has five carbon atoms?
the straight-chain alkane has at least one double or triple bond, but the cycloalkane has only single bonds.
the straight-chain alkane has only single bonds, but the cycloalkane has at least one double or triple bond.
the straight-chain alkane has two more hydrogen atoms than the cycloalkane.
the straight-chain alkane has two fewer hydrogen atoms than the cycloalkane.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
How does a straight-chain alkane that has five carbon atoms differ from a cycloalkane that has five...
Questions