
Chemistry, 09.11.2019 21:31 natfloresm13
Salicylic acid (c7h6o3) reacts with acetic anhydride (c4h6o3) to form acetylsalicylic acid (c9h8o4).
2c7h6o3(aq) + c4h6o3(aq) mc002-1.jpg 2c9h8o4(aq) + h2o(l)
what is the limiting reactant if 70.0 g of c7h6o3 and 80.0 g of c4h6o3 react?
a water
b salicylic acid
c acetic anhydride
d acetylsalicylic acid

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2
You know the right answer?
Salicylic acid (c7h6o3) reacts with acetic anhydride (c4h6o3) to form acetylsalicylic acid (c9h8o4)....
Questions



Mathematics, 17.07.2020 06:01


Mathematics, 17.07.2020 06:01


Mathematics, 17.07.2020 06:01



Mathematics, 17.07.2020 06:01

Computers and Technology, 17.07.2020 06:01


Mathematics, 17.07.2020 06:01







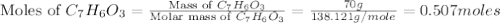
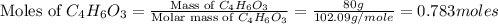
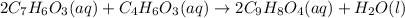
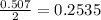 mole of acetic anhydride
mole of acetic anhydride

