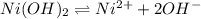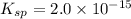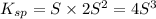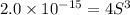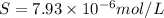Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Calculate the molar solubility of ni(oh)2 in water. use 2.0 * 10^-15 as the solubility product const...
Questions



History, 19.07.2019 01:40

History, 19.07.2019 01:40

Advanced Placement (AP), 19.07.2019 01:40


Health, 19.07.2019 01:40



Geography, 19.07.2019 01:40

Mathematics, 19.07.2019 01:40



English, 19.07.2019 01:40

History, 19.07.2019 01:40





Computers and Technology, 19.07.2019 01:40

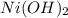 in water
in water 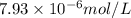 .
.