
Chemistry, 20.04.2021 14:00 julianbeaver76
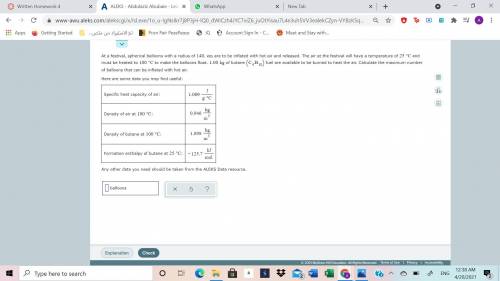
At a festival, spherical balloons with a radius of 140cm are to be inflated with hot air and released. The air at the festival will have a temperature of 25 C and must be heated to100 C to make the balloons float. 1.00kg of butane(C4H10) fuel are available to be burned to heat the air. Calculate the maximum number of balloons that can be inflated with hot air.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
At a festival, spherical balloons with a radius of 140cm are to be inflated with hot air and release...
Questions

Mathematics, 26.10.2021 22:20


History, 26.10.2021 22:20



English, 26.10.2021 22:20

Mathematics, 26.10.2021 22:20

History, 26.10.2021 22:20


Chemistry, 26.10.2021 22:20


History, 26.10.2021 22:20

Mathematics, 26.10.2021 22:20

Mathematics, 26.10.2021 22:20

Computers and Technology, 26.10.2021 22:20

Chemistry, 26.10.2021 22:20




Computers and Technology, 26.10.2021 22:20



